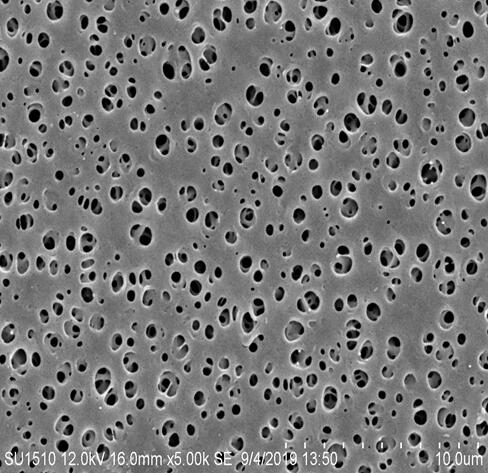
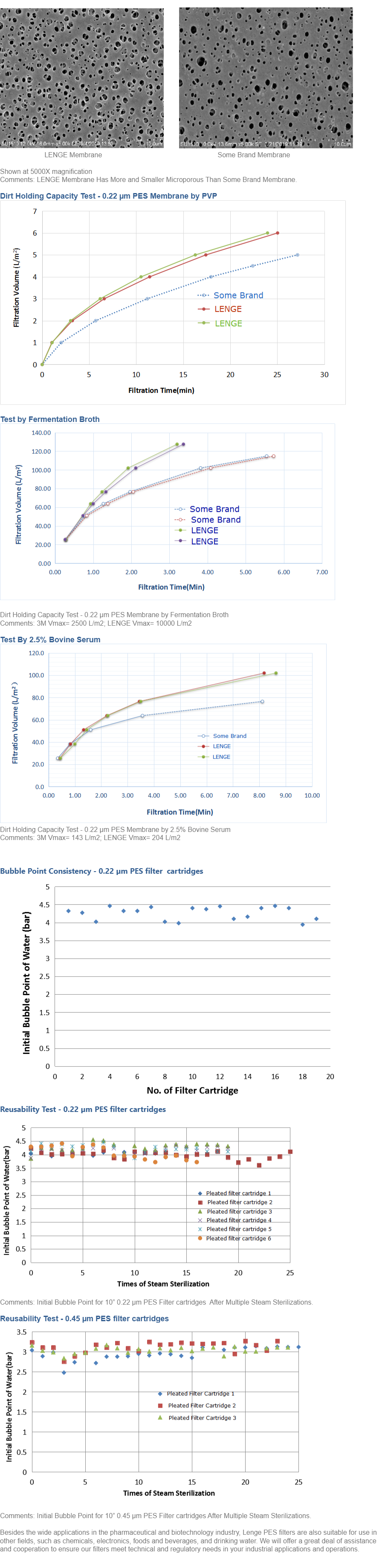
Polyethersulfone (PES) Flat Membrane
The PES membranes for making cartridge filters at Lenge were developed and commercialized using its owned proprietary membrane manufacturing technologies in clean rooms in a state-of-the-art facility.
All raw materials used for membrane production were suitable for manufacturing pharmaceutical grade filters, and the production lines were designed and manufactured by a world-class membrane machine manufacturer. The operating conditions and procedures in the membrane manufacturing were computer controlled so that the membranes produced have precise pore sizes and desired mechanical and filtration performance properties. Lenge offers the following pore size rated PES filters with various filter lengths (0.5 - 40 inches) to match the dimension and sealing requirements for most cartridge housings currently available:
• 0.1 µm
• 0.22 µm (fully bacteria retentive)
• 0.45 µm
• 0.65 µm
The Lenge PES membranes are highly asymmetric with a distinct uniform integral retention layer. The membranes have a high surface porosity and gradually increased pore size distribution in the direction facing upstream of filtration. Therefore, the membranes have a capability of retaining various sized particulates and biological species. The Lenge PES membrane filters have the following distinguished characteristics:
• High flux
• High capacity
• High mechanical strength
• High porosity
• Low protein binding and adsorption
• Low leaching
• Low endotoxic
The validation of bacterial B. diminuta retention for 0.22 µm rated pharmaceutical grade cartridges was performed in accordance with American Society of Testing and Materials (ASTM) method F838-05. Using this test methodology, 0.22 µm rated pharmaceutical grade filters retained greater than 1 x 107 CFU of B. diminuta per cm² of effective filter surface area.
Features and Benefits:
• Complies with United States Pharmacopeia (USP) Biological Reactivity Test, In Vivo <88> for biosafety, cytotoxicity, and hemolysis testing.
• For 0.1.0.22µm membrane,Lot samples retain a minimum of 10E7 cfu/cm2 of Brevundimonas diminuta(ATCC19146) per modified ASTM F838, current revision.
• The optimized built-in Highly asymmetric prefilter layer for maximum flow and throughput performance.
• Hydrophilic membrane that wets out quickly and completely resulting in fast filtration with superior flow rates and high throughputs. Easily wet for reliable integrity testing.
• Very high compatibility over the whole pH range plus very low protein binding to ensure the maximum transmission of the active ingredients.
• Compatible with EtO, gamma irradiation, and SIP/autoclave methods of sterilization.
Sealing methods:
• Mechanical
• Heat
• Ultrasonic
• RF welding
• Insert molding
- Country of origin
- China